Atoms are supposed to be stable: there was supposed to be a law called the law of conservation of matter. That means that atoms can’t appear or disappear. They just combine or break apart.
For example, here is the decomposition of ammonium dichromate. Nothing is gained or loss – just reorganized.
But in the late 1800s, Henri Becquerel, and Pierre and Marie Curie, discovered the phenomenon of radioactivity. Some atoms were discovered to give off invisible energy!?
And in 1909 Ernest Rutherford discovered one of the basic mechanisms behind radioactivity: the mass of some atoms changed over time. Seemingly, some matter vanished? And other atoms appeared that literally were not there before.
Magic? Impossible?! But the results were verified – anyone could observe this once they knew what to look for.
This was the first clue that our classical laws of physics were incomplete! It became our doorway into modern physics.
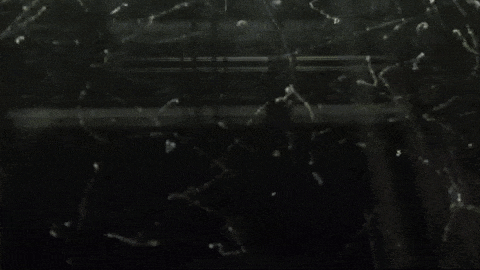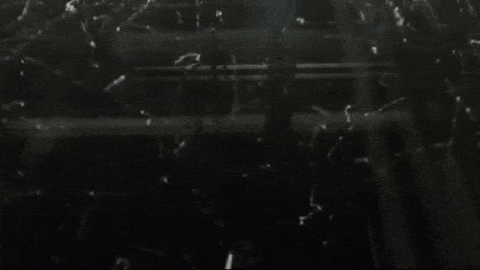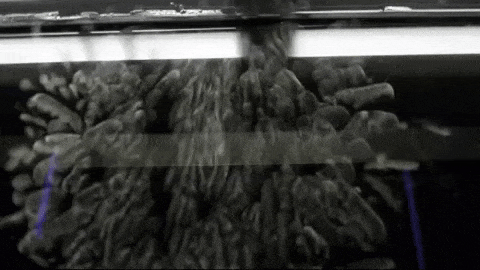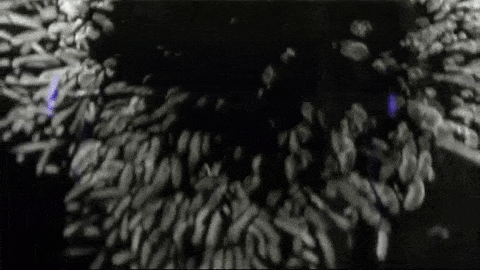
Image below: “This is a Diffusion Cloud Chamber used for public demonstrations at the Museum of Technology in Berlin. The first part shows the alpha and beta radiation occurring around us all the time, thanks to normal activity in the atmosphere. Then a sample of Radon 220 (half-life 55 sec) is inserted into the chamber and all hell breaks loose as an alpha-decay party ensues.”
http://physicsfootnotes.com/radon-cloud-chamber/
https://www.youtube.com/watch?v=Efgy1bV2aQo
The Nucleus
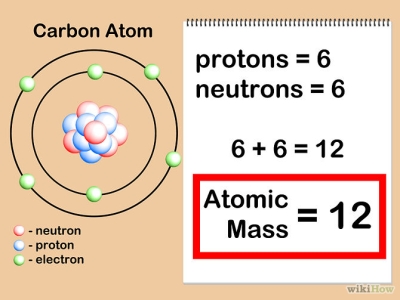
Atoms are composed of sub-atomic particles.
We model atoms as if electrons, protons and neutrons were solid objects.
We draw the electrons orbiting around the nucleus as if they were planets orbit around a star.
*
And we count the number of P + N, and add them together to get the mass of the atom (atomic mass)
We don’t add the mass of the e-, because they are so small.
It would take almost 2,000 e to have the same mass as one p.
Misconception: Atoms are like a solar system?
Electrons are not solid objects.
They usually exist as if they are clouds of energy surrounding the nucleus.
Darker parts of the cloud are regions where the electron is more likely to be found, if we probe for it. Lighter parts of the cloud are regions where the electron is less likely to be found.
What’s amazing about the images below, is that each represents only one electron.
Those double-barreled “p” orbitals aren’t showing two electrons – that is the probability cloud of one electron!
The “f” orbitals are showing 6 or 8 different electrons – each of those is, again, a single electron.
Later we can give some simple analogies why this is so, and how this, in the big picture, can make sense. But for now we note a few take-home points:
-
atoms are not solid
-
atoms are made of smaller components
-
each of these components, in many simple chemical reactions, acts as if they are solid particles
-
so it is generally useful to draw them as solid objects
-
most atoms are stable (they last forever)
-
they are not “really” solid: their more correct, probability cloud nature becomes important when we study advanced chemistry. This lets us discover how three dimensional molecules really bond together.
-
some atoms are not stable – some atoms can spontaneously break apart or transform into other atoms, and this leads into radioactivity.
Binding Energy and Nuclear Forces
Opposites charges attract. Like charges repel.
So how can the nucleus be full of protons?
They should repel each other!
Mind blown! Atomic nuclei should not exist at all!
Protons do repel each other…So atoms don’t exist?!
Okay, atoms do exist… so we just must have missed something.
The Doctor is asking us to think it through…
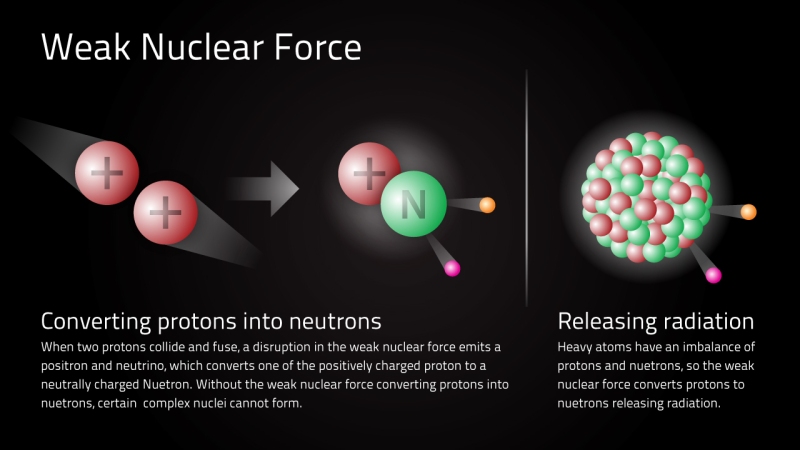
If protons repel each other, yet are bound together, then there must be some other force of nature! NUCLEAR FORCES!
These are forces that act over Very Short Distances.
They only have measurable strength within the distances the size of an atom’s nucleus, or shorter.
On distance scales larger than that, these forces are effectively zero.
The strong nuclear force
The weak nuclear force
Radioactivity
Towards the end of the 19th century, minerals were found that would darken a photographic plate even in the absence of light. This phenomenon is now called radioactivity.
Marie and Pierre Curie isolated two new elements that were highly radioactive; they are now called polonium and radium.
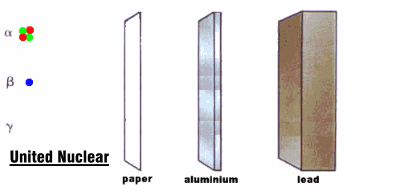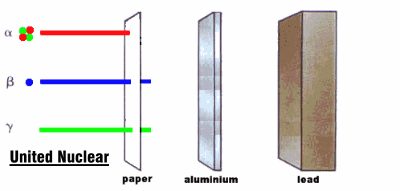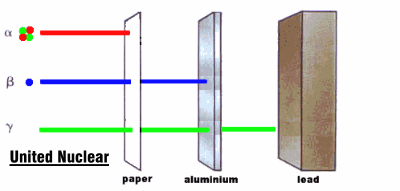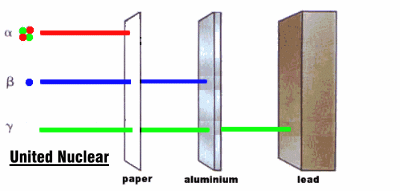
Radioactive rays were observed to be of three types:
1. Alpha rays, which could barely penetrate a piece of paper
2. Beta rays, which could penetrate 3 mm of aluminum
3. Gamma rays, which could penetrate several centimeters of lead
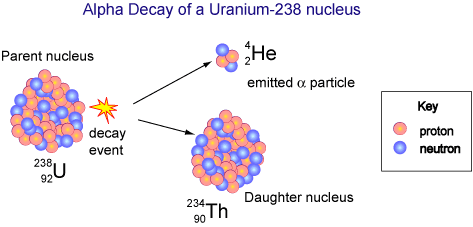
We now know that alpha rays are helium nuclei, beta rays are electrons, and gamma rays are a kind of electromagnetic radiation.
Alpha Decay
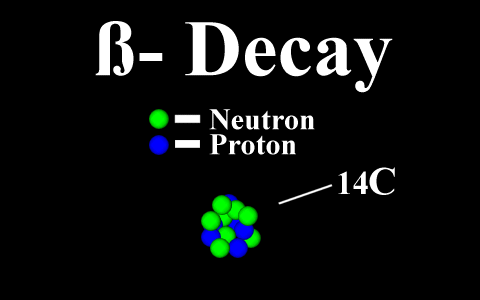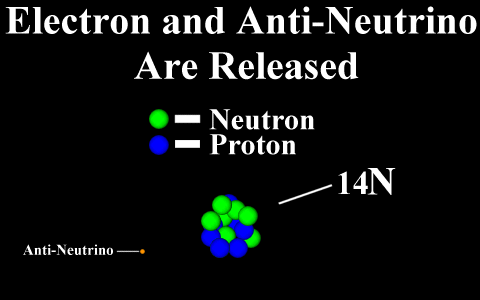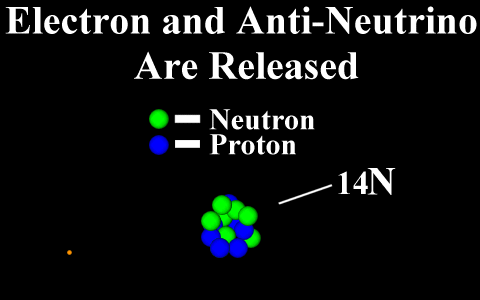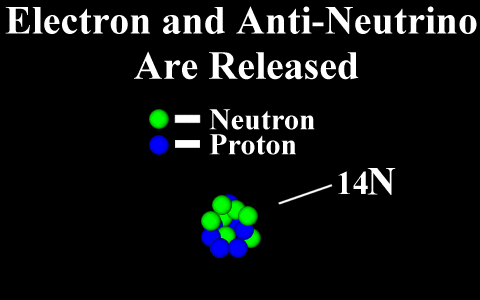
Beta Decay
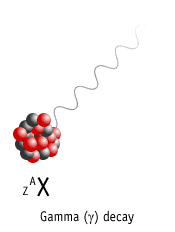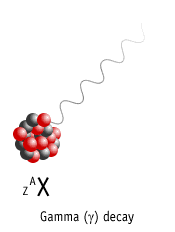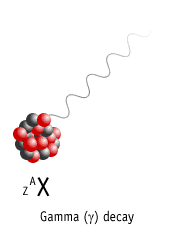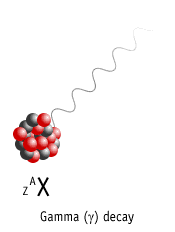
Gamma Decay
Here a nucleus decays from an excited state into a lower energy state, by emitting a gamma ray.
Element is not changed to another element (no nuclear transmutation)
Conservation of Nucleon Number
Connections with Earth Science
The Earth is 4.5 billion years old, yet it still has not cooled down yet. Why not? The Earth’s interior has massive amounts of radioactive metal! See –Why is the Earth still hot?
On Earth, the production of new helium is result of radioactive decay. Helium is found in large amounts in minerals of uranium and thorium. See – Helium cycle
Nuclear power and cancer
In theory, during an accident, radiation released from nuclear power plants can increase the background rates of cancer, perhaps dramatically. It has long been expected by opponents of nuclear power that it’s use would be highly dangerous.
Yet in the 60 years of its use, the number of actual accidents, Soviet designed tragedies like Chernobyl (an event in a class by itself, due to deliberate malfeasance), and even Fukushima Daiichi, the tsunami-damaged nuclear reactor site, have caused far less damage and death than coal, oil and other sources of power.
Surprisingly, simply burning coal releases more radiation into the environment than running a nuclear reaction. Similarly, getting into an airplane to fly away from Fukushima Daiichi caused thousands of Japanese citizens to be exposed to even more ionizing radiation than if they had simply stayed at home.
Airplane flights make one rise above most of the atmosphere, thereby increasing one’s exposure to natural background radiation from space.
There is also the intriguing phenomenon of radiation hormesis:
Radiation hormesis is the hypothesis that low doses of ionizing radiation (just above natural background levels) are beneficial. Low level radiation apparently activates repair mechanisms that protect against disease, that are not activated in absence of ionizing radiation.
The reserve repair mechanisms are hypothesized to be sufficiently effective as to not only cancel the detrimental effects of ionizing radiation – but also inhibit disease not related to radiation exposure. This counter-intuitive hypothesis has captured the attention of scientists and public alike in recent years.
Radiation hormesis. (2016, December 10). In Wikipedia, The Free Encyclopedia. Retrieved 18:44, February 2, 2017
Radiation hormesis (Wikipedia)
There is no environment without some level of background radioactivity. What society needs to do is become familiar with the statistics, so it can make informed choices on how much power to generate/consume, and where this power should come from.
Coal releases more radioactivity than nuclear power
Learning Standards
SAT Subject Test: Physics
Quantum phenomena, such as photons and photoelectric effect
Atomic, such as the Rutherford and Bohr models, atomic energy levels, and atomic spectra
Nuclear and particle physics, such as radioactivity, nuclear reactions, and fundamental particles
Relativity, such as time dilation, length contraction, and mass-energy equivalence
A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas (2012)
Electromagnetic radiation can be modeled as a wave of changing electric and magnetic fields or as particles called photons. The wave model is useful for explaining many features of electromagnetic radiation, and the particle model explains other features. Quantum theory relates the two models…. Knowledge of quantum physics enabled the development of semiconductors, computer chips, and lasers, all of which are now essential components of modern imaging, communications, and information technologies.
Massachusetts Science and Technology/Engineering Curriculum Framework 2006
Chemistry: Atomic Structure and Nuclear Chemistry
Atomic models are used to explain atoms and help us understand the interaction of elements and compounds observed on a macroscopic scale. Nuclear chemistry deals with radioactivity, nuclear processes, and nuclear properties. Nuclear reactions produce tremendous amounts of energy and lead to the formation of elements.
2.1 Recognize discoveries from Dalton (atomic theory), Thomson (the electron), Rutherford (the nucleus), and Bohr (planetary model of atom), and understand how each discovery leads to modern theory.
2.2 Describe Rutherford’s “gold foil” experiment that led to the discovery of the nuclear atom. Identify the major components (protons, neutrons, and electrons) of the nuclear atom and explain how they interact.
2.3 Interpret and apply the laws of conservation of mass, constant composition (definite proportions), and multiple proportions.
2.4 Write the electron configurations for the first twenty elements of the periodic table.
2.5 Identify the three main types of radioactive decay (alpha, beta, and gamma) and compare their properties (composition, mass, charge, and penetrating power).
2.6 Describe the process of radioactive decay by using nuclear equations, and explain the concept of half-life for an isotope (for example, C-14 is a powerful tool in determining the age of objects).
2.7 Compare and contrast nuclear fission and nuclear fusion.